Institute of Pharmacology and Toxicology
Research
As a research institute we focus on translational medicine to elucidate disease mechanisms, to identify new drug targets and to develop novel therapeutic strategies.
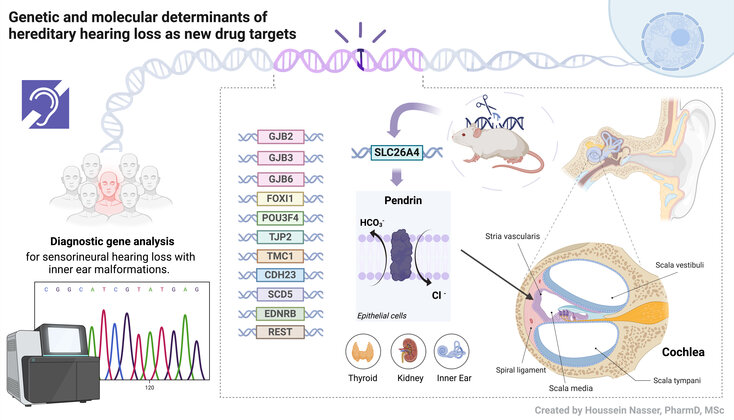
The main research focus of the Institute of Pharmacology and Toxicology is the understanding of genetic and molecular mechanisms of syndromic (Pendred syndrome) and non-syndromic malformative hearing loss. In collaboration with the Department of Otolaryngology at SALK (Prof. G. Rasp and Dr. S. Rösch) we have recruited the first Austrian cohort of patients with hearing loss associated with an enlarged vestibular aqueduct (EVA), one of the most common causes of sensorineural hearing loss (Roesch et al. 2018). The SLC26A4 gene, coding for the anion exchanger pendrin, is frequently mutated in the context of Pendred syndrome and non-syndromic EVA. Goal of the research is the precise understanding of the molecular derangements leading to loss of function of pathogenic variants of the pendrin protein, including structural alterations, cellular mis-localization, aberrant turnover and altered gene transcription. The possible contribution of additional genes, including but not limited to connexins GJB2, GJB3 and GJB6, as well as transcription factors such as FOXI1 and POU3F4, in determining malformative hearing loss is also investigated. We also strive to identify novel hearing loss gene and gene modifiers that could explain the disease phenotype in undiagnosed patients. Ultimate goal is to identify novel molecular targets, pathways and strategies for the development of future therapies for hearing loss.
Key publications
Jonard L, Brotto D, Moreno-Pelayo MA, del Castillo I, Kremer H, Pennings R, Caria H, Fialho G, Boudewyns A, Van Camp G, Oldak M, Ozieblo D, Deggouj N, De Siati R, Gasparini P, Girotto G, Verstreken M, Dossena S, Roesch S et al. Genetic Evaluation of Prelingual Hearing Impairment: Recommendations of an European Network for Genetic Hearing Impairment. Audiology Research. 2023; 13(3):341-346.
Bernardinelli E, Roesch S, Simoni E Marino A, Rasp G, Astolfi L, Sarikas A and Dossena S. Novel POU3F4 variants identified in patients with inner ear malformations exhibit aberrant cellular distribution and lack of SLC6A20 transcriptional upregulation. Front Mol Neurosci. Sept 2022.
Roesch, S; Bernardinelli, E; Nofziger, C; Tóth, M; Patsch, W; Rasp, G; Paulmichl, M; Dossena, S; Functional Testing of SLC26A4 Variants-Clinical and Molecular Analysis of a Cohort with Enlarged Vestibular Aqueduct from Austria. Int J Mol Sci. 2018; 19(1):
Pera A, Dossena S, Rodighiero S, Gandía M, Bottà G, Meyer G, Moreno F, Nofziger C, Hernández-Chico C, Paulmichl M. Functional assessment of allelic variants in the SLC26A4 gene involved in Pendred syndrome and nonsyndromic EVA. Proc Natl Acad Sci U S A. 2008;105:18608-18613.
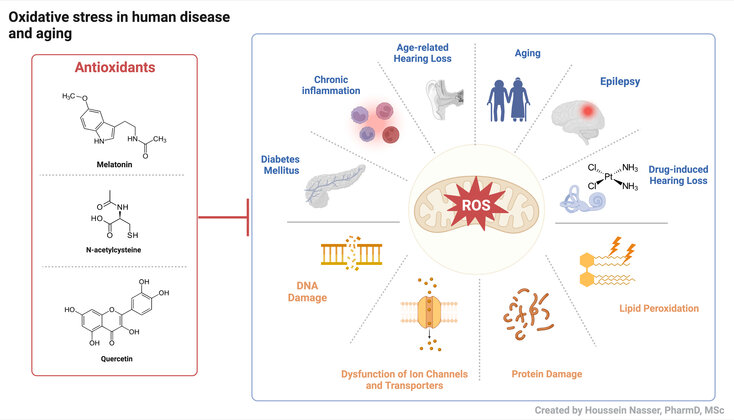
Reactive Oxygen Species (ROS) are highly reactive chemicals normally produced by the cellular metabolism or following exposure to environmental stressors and xenobiotics. When ROS accumulate beyond the endogenous antioxidant capacity of biological systems, a condition called oxidative stress (OS) ensues. OS underlies several pathophysiological conditions including diabetes mellitus, hearing loss, epilepsy, and aging. The scope of the research is the identification of cellular and molecular targets of OS, with emphasis on ion channel and transporters, and exploring the use of antioxidants to counteract the detrimental effect of OS on a cellular level.
Key publications
Spinelli S, Remigante A, Liuni R, Mantegna G, Legname G, Marino A, Morabito R, Dossena S. Oxidative stress-related cellular aging causes dysfunction of the Kv3.1/KCNC1 channel reverted by melatonin. Aging Cell. 2024 May 9:e14185.
Remigante A, Spinelli S, Zuccolini P, Gavazzo P, Marino A, Pusch M, Morabito R, Dossena S. Melatonin protects Kir2.1 function in an oxidative stress-related model of aging neuroglia. Biofactors. 2023 Dec 14
Remigante A, Spinelli S, Trichilo V, Loddo S, Sarikas A, Pusch M, Dossena S, Marino A, Morabito R. d-Galactose induced early aging in human erythrocytes: Role of band 3 protein. J Cell Physiol. 2022 Feb;237(2):1586-1596.