Follow-up study highlights the potential of sprifermin therapy for knee osteoarthritis
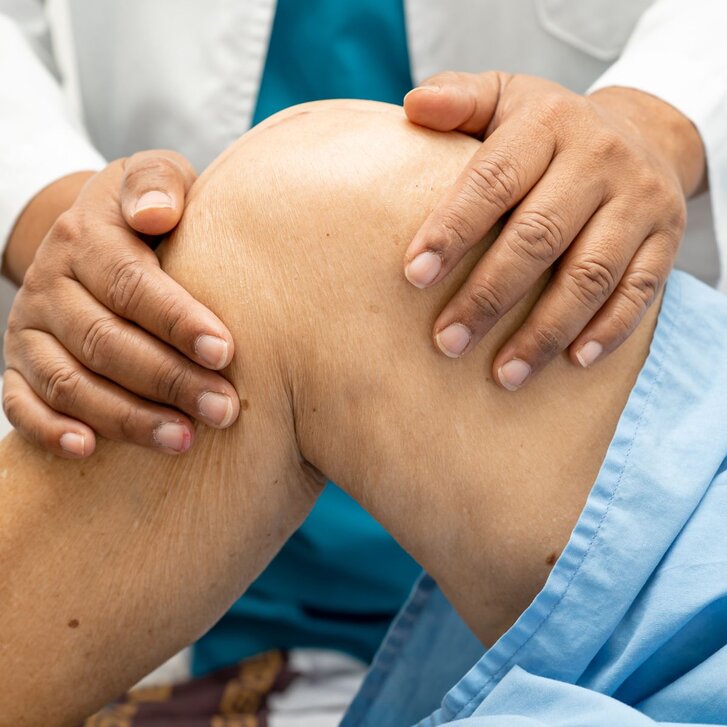
To date, no medication with proven structural efficacy for knee osteoarthritis has been approved for clinical use. The FORWARD study (RCT; Phase 2B) focuses on the long-term effectiveness of sprifermin, an anabolic cartilage drug with the potential to structurally treat knee osteoarthritis. Univ.-Prof. Dr. Felix Eckstein, head of the Musculoskeletal Imaging Research Program at Paracelsus Medical University (PMU) in Salzburg, and his team demonstrated the potential of sprifermin in a follow-up study. Their findings were published in the article “Unbiased analysis of knee cartilage thickness change over three years after sprifermin vs. placebo treatment - A post-hoc analysis from the phase 2B FORWARD study,” recently featured in the journal Osteoarthritis and Cartilage Open (see: https://doi.org/10.1016/j.ocarto.2024.100513).
Professor Eckstein explains: “The goal of this study was to assess whether the cartilage generated during treatment remains stable over an extended period after therapy ends, indicating sustained efficacy of sprifermin. Over the course of two years of treatment, we demonstrated not only a halt in cartilage loss but also a significant increase in cartilage thickness with sprifermin.”
The study involved 549 patients with radiographically confirmed knee osteoarthritis, who received either a placebo or one of four different doses of sprifermin via intra-articular injection. After the treatment phase, the patients were monitored for an additional three years. During the five-year combined treatment and follow-up period, MRI scans were performed to measure knee cartilage thickness using specialized medical-grade software (Chondrometrics 2.0). The images were assessed by experienced evaluators, who were blinded to the order of the scans.
“The findings revealed that the cartilage formed during sprifermin therapy did not degrade faster than ‘normal’ arthritic cartilage over the three years post-treatment, and the sprifermin-induced benefits seen at the end of treatment were fully maintained. This suggests that the cartilage formed during the treatment phase remains structurally stable and mechanically functional over the long term. These results underscore sprifermin’s potential to positively influence the disease course of knee osteoarthritis and potentially enhance patients' quality of life in the long run,” Eckstein summarizes.
Sprifermin is still under clinical development and is not yet approved for medical use.
---